CONRAD is a boutique hybrid of academia, non-profit, pharmaceutical/ biomedical R&D, and sociobehavioral research/human-centered design encompassing end-to-end expertise. Our holistic approach to our work makes our products, technologies, and research solutions more innovative, cutting edge and likely to add real value in underserved and low-resource settings.
What We Do
 Translational & preclinical research
Translational & preclinical research
Mucosal susceptibility to HIV; novel mucosal safety endpoints; PK & efficacy models; novel adherence biomarkers
 Product development & quality assurance
Product development & quality assurance
Pharmaceutical & combination drug-device product development; technology transfer, set up, validation, and quality monitoring of cGMP manufacturing of clinical supplies
 IND enabling
IND enabling
Testing plan design, implementation & oversight for pharmaceuticals, medical devices & combination drug-device products
 Adherence support & acceptability testing
Adherence support & acceptability testing
End user research to support product development, uptake, and adherence
 Regulatory submissions
Regulatory submissions
Documentation to support IND, IDE, NDA, and other regulatory requirements
 Clinical studies
Clinical studies
Exploratory, phase I, II, and III clinical trials, and demonstration/implementation studies
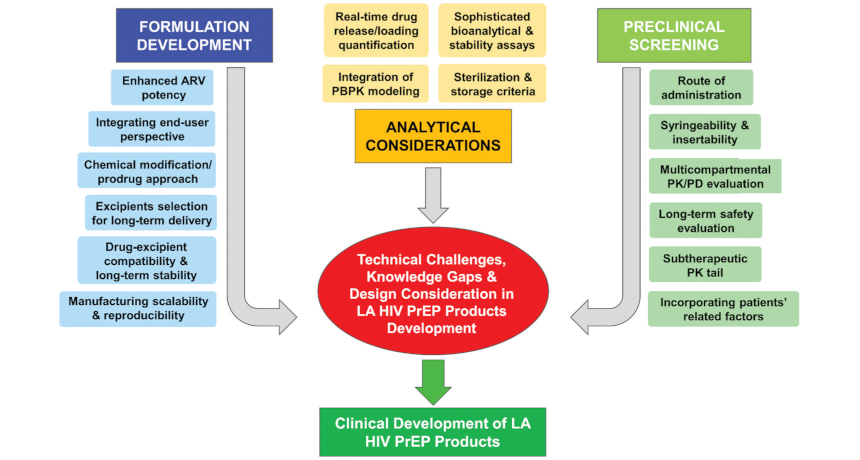
From discovery to preclinical and early clinical testing, clinical trials (focusing on Safety, PK, PD, acceptability, and effectiveness), human-centered design and end-user research, and regulatory strategy, all the way through to implementation, CONRAD is committed to designing safe, affordable, and user-friendly solutions to global health challenges.
Highlights
 First-in-Class Intravaginal Ring containing Tenofovir and Levonorgestrel for prevention of HIV and unintended pregnancy
First-in-Class Intravaginal Ring containing Tenofovir and Levonorgestrel for prevention of HIV and unintended pregnancy
More
 Vuka+: a novel smartphone-based PrEP adherence support intervention for adolescent girls and young women
Vuka+: a novel smartphone-based PrEP adherence support intervention for adolescent girls and young women