Despite ongoing efforts to increase treatment and prevention of HIV, poor and inconsistent adherence to daily pre-exposure prophylaxis has emerged as a key barrier to achieving the UNAIDS (The Joint United Nations Programme on HIV/AIDS) global targets for reducing annual HIV infections and AIDS-related deaths. There are many factors that present significant challenges to adherence to the primary route for currently marketed antiretrovirals, daily oral pills, including social acceptance of a regimen, side effects, and pill burden and fatigue.
As part of our mission to conceive novel products that truly address end-user wants and needs, CONRAD invests significant effort into overcoming many conventional treatment barriers for underserved populations in need of antiretroviral therapies. The development of long-acting, potent antiretrovirals thanks in part to parallel advancement of controlled release technologies enables drug delivery systems to potentially provide extended dosing intervals capable of overcoming some of the challenges that result in suboptimal drug adherence during daily oral dosing.
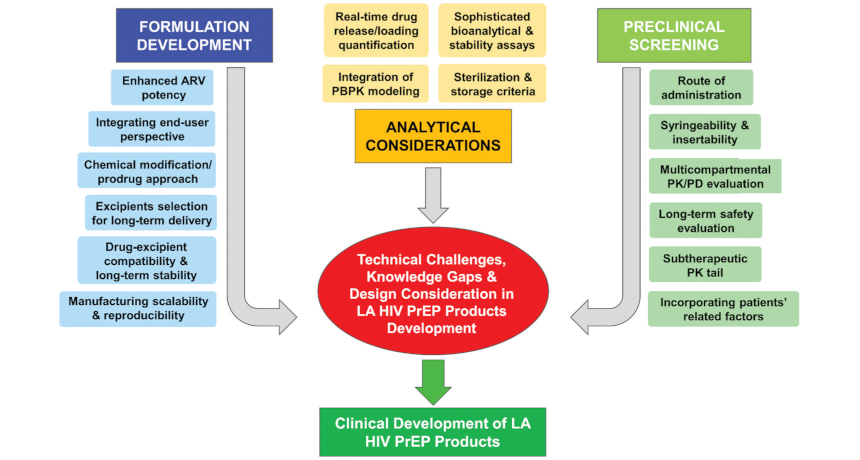
Staying conscious of the key technological challenges, knowledge gaps, and preclinical testing considerations that still have yet to be fully overcome, CONRAD is developing a number of innovative long-acting systems that keenly integrate end-user perspectives:
- CAB injectable silica matrix hydrogel depot
- CAB multi-pellet system
- TAF suspension patch
- 3D printed microarray patch for the controlled and delayed release of HIV broadly neutralizing antibodies (bnAbs)
The HIV prevention field continues to evolve more rapidly than ever before, presenting both new opportunities and new challenges in developing next-generation long-acting HIV prevention options. Requirements for implementing viable long-acting PrEP products in resource-limited settings remain particularly challenging, requiring a proactive approach open to flexible product modifications during the design and testing phases of these promising new candidates. If these new technologies and design techniques can be successfully translated, next-generation long-acting PrEP that is safe, affordable, and highly effective will offer significant potential to curb the HIV pandemic.